Abstract
Lenalidomide is a highly active treatment for multiple myeloma. We and others have previously shown that lenalidomide targets Ikaros (IKZF1) and Aiolos (IKZF3) for ubiquitination and degradation via the cereblon (CRBN) E3 ubiquitin ligase. Depletion of these lymphoid transcription factors selectively inhibits growth of multiple myeloma cells. In contrast to human cells, mouse cells are insensitive to lenalidomide due to a single amino acid change in the murine CRBN (mCRBN) protein (isoleucine instead of valin at position 391). Therefore, lenalidomide could so far not be studied in syngenic mouse multiple myeloma models. The mouse MOPC-315.BM cell line engrafts in the bone marrow of BALB/c mice and induces multiple myeloma after intravenous injection (Riedel et al., PLoS one 2012 and Hofgaard et al., PLoS one 2012). We aimed to generate a lenalidomide-sensitive mouse model by retroviral expression of a mutant murine Crbn where the isoleucine at position 391 is replaced by valin (mCrbnI391V) in MOPC-315.BM cells.
Western blot analyses showed that MOPC-315.BM multiple myeloma cells express IKZF1 and IKZF3 proteins at a high level as compared to non-myeloma cell lines. Expression of a dominant negative IKZF3 isoform that inactivates both, IKZF3 and IKZF1, inhibited proliferation of MOPC315.BM cells, demonstrating that these cells depend on IKZF3 and IKZF1. In unmodified or mCrbn -overexpressing MOPC-315.BM cells even high concentrations (>10 µM) of lenalidomide did not induce degradation of IKZF1 and IKZF3 and consistently had no effect on proliferation. In contrast, we observed effective degradation of IKFZ1 and IKZF3 in MOPC-315.BM expressing mCrbnI391V and these cells were highly sensitive to lenalidomide, pomalidomide, and thalidomide. Having shown that expression of mCrbnI391V sensitizes mouse multiple myeloma cells in vitro, we sought to investigate whether this is also true in vivo .
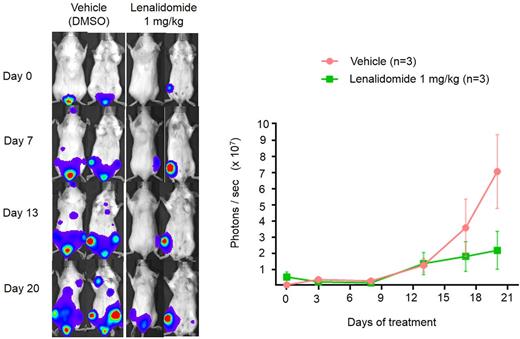
For that purpose, we injected mCrbnI391V-expressing MOPC-315.BM cells in BALB/c mice. Luciferase expression of MOPC-315.BM cells allowed us to monitor tumor growth non-invasively by bioluminescense at different time points. Similar to the unmodified MOPC-315.BM cells, we detected mCrbnI391V-expressing MOPC-315.BM in the bone between days 12 to 19 by bioluminescense and continuous growth of the lesions until mice became sick around day 40. This demonstrated that expression of mCrbnI391V did not affect the potential of MOPC-315.BM to induce multiple myeloma in vivo. We next treated the mice with different dosages and schedules of lenalidomide by intraperitoneal injection when tumor engraftment was clearly measurable by bioluminescense in all mice. Lenalidomide at a high concentration of 25 mg/kg given twice weekly had only weak effects on tumor growth. In contrast, daily treatment with low concentrations (1 mg/KG) effectively inhibited tumor growth, demonstrating that continuous inactivation of IKZF1 and IKZF3 is necessary for lenalidomide activity in vivo .
In conclusion, our results demonstrate that mouse multiple myeloma cells can be sensitized to lenalidomide by expression of mCrbnI391V in vitro and in vivo . This model will allow for the first time to test drug combinations including lenalidomide in a syngenic mouse model. Furthermore, it serves as a proof-of-concept for the generation of transgenic lenalidomide-sensitive mouse models for multiple myeloma and other hematologic malignancies in the future.
Kronke: Celgene: Honoraria, Other: Travel Support; Takeda: Consultancy, Other: Travel Support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal